Reactive monomers and polymers
Synthesis and application of new reactive monomers and corresponding polymers
Sulfur-based monomers
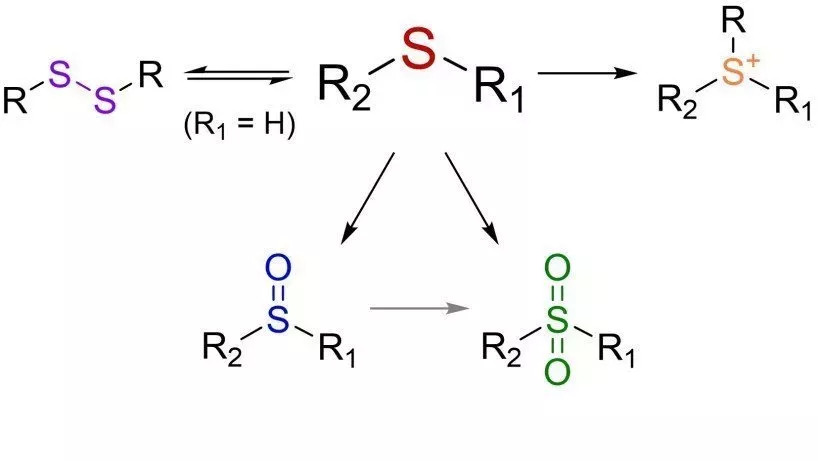
Sulfur based monomers (Illustration: Johannes C. Brendel)
Sulfur compounds offer a multitude of modification possibilities in organic synthesis, for example the oxidation of thioethers, the formation of disulfide bridges or the formation of cationic sulfonium ions. Due to their inherently high refractive index, such materials find applications in optical components, among others, but the variety of modifications also opens up potential in biomedical areas. Our general interest is the development of sulfur-containing monomers and their polymerization by different methods, e.g. radical polymerization or condensation or addition reactions. Thereby, properties such as the molar mass should be precisely adjusted and functional end groups should be introduced in a targeted manner. Furthermore, the sulfur groups enable various subsequent modifications to these polymers, whereby properties can be specifically adapted and the range of applications expanded.
Oxidation-responsive micelles for targeted drug release
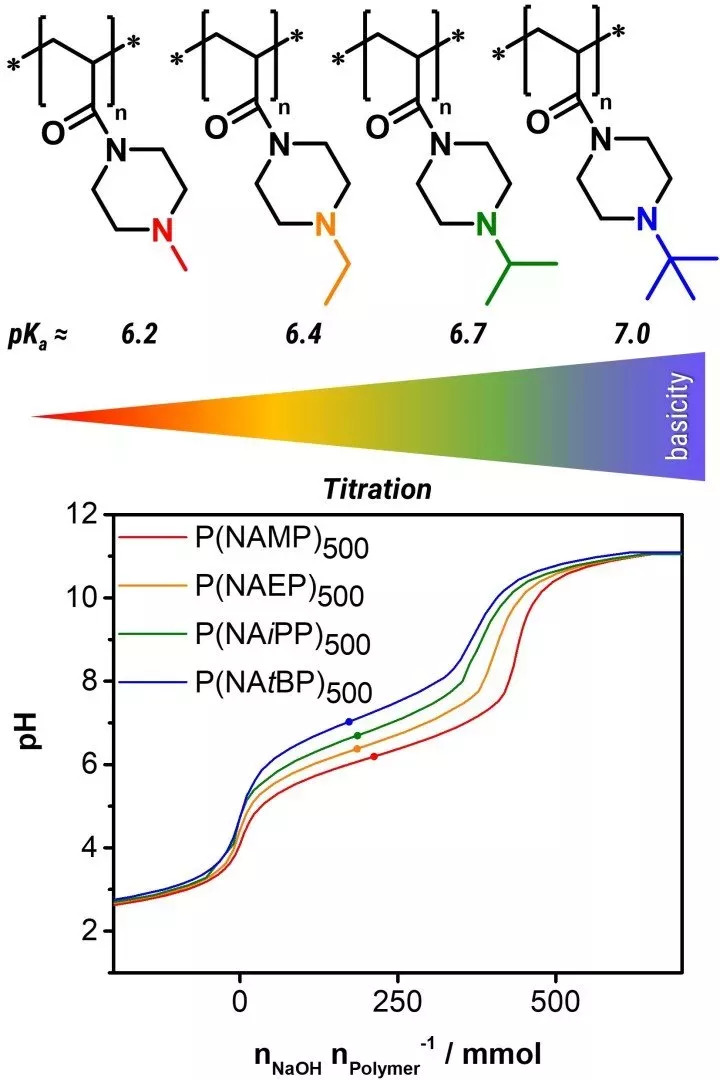
pH-responsive polymers (Illustration: Johannes C. Brendel)
Polymers with reversibly protonable groups, such as amines or carboxylic acids, usually react very strongly to changes in pH. When a proton is added or split off, they become correspondingly ionically charged and are therefore also known as weak polyelectrolytes. Of particular interest for our research is the physiologically relevant pH value range from neutral (7.4) to slightly acidic (approx. 4-5), which is passed through, for example, during endocytotic uptake into the cell. For this purpose we are developing, among other things, novel monomers or polymers that react in this range. N-acryloyl-modified piperazine derivatives represent a promising material, whose basicity can be varied depending on the substituent on the amino group. In addition, the reactive acrylamide structure enables fast but controlled radical polymerization by means of the RAFT process (RAFT: reversible addition-fragmentation chain transfer) up to high molecular weights (> 100,000 g/mol).

